Claim:
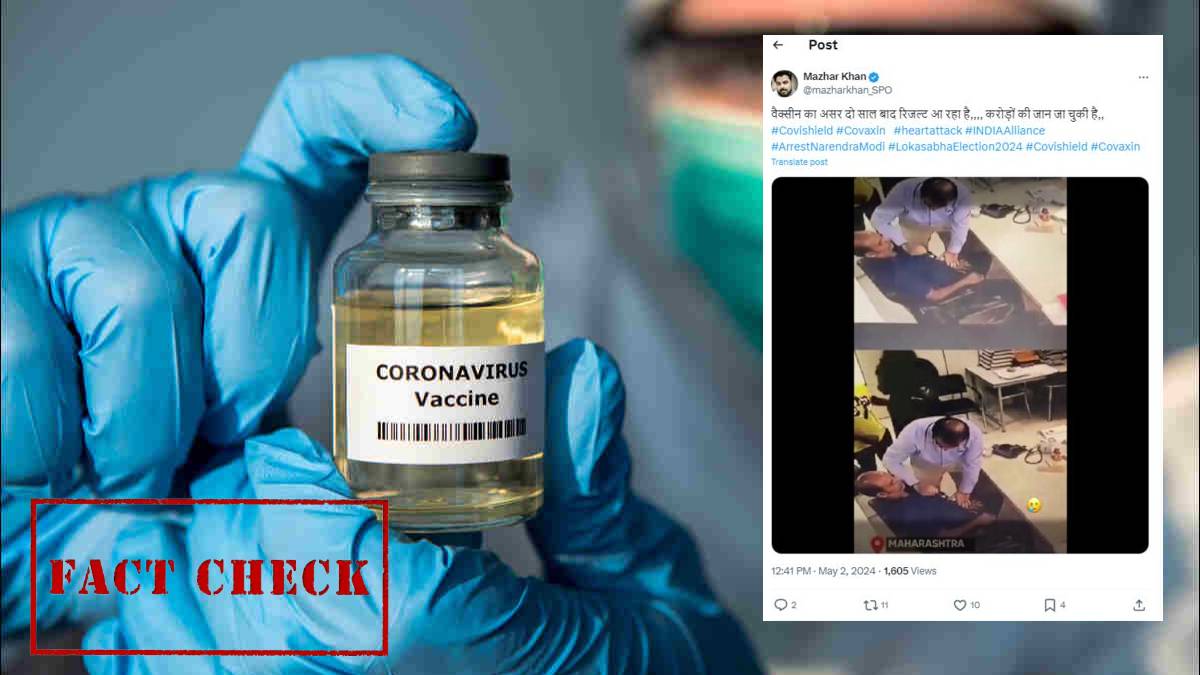
An X user has shared a post which claims Covaxin COVID vaccine has caused the death of crores of people across India.

Investigation:
Is it true that Covaxin can cause death even after two years of vaccination?
No, Covaxin COVID vaccine cannot cause death two years after vaccination. As of the latest available information, there is no conclusive evidence linking Covaxin, developed by Bharat Biotech, to an increased risk of heart attacks or death occurring two years post-vaccination. Vaccines, including Covaxin, undergo rigorous testing for safety and efficacy before approval and continued monitoring even after approval.
Some adverse events following vaccination can occur. But, these are generally observed within a short period after vaccination, and long-term severe side effects are extremely rare. Covaxin was approved for emergency use by the WHO, after a complete risk-benefit analysis. Moreover, vaccines are the most important tool in the fight against any disease. We must also remember we were combating a pandemic at that time. As we have stated it earlier, vaccines have saved lives and have helped in controlling the spread of disease.
It’s important to consider that heart attacks and other cardiovascular events can occur due to a variety of factors, such as underlying health conditions, lifestyle, and genetic predispositions. These events happening two years post-vaccination are unlikely to be directly related to the vaccine itself.
Were there clinical trials of the Covaxin?
Yes, Covaxin COVID vaccine underwent extensive clinical trials. In June 2020, the Ministry of Health and Family Welfare in India gave permission to start Phase I and II human trials for Covaxin after preclinical studies showed it was safe and produced a strong immune response in animals. Bharat Biotech conducted Phase I and Phase II trials involving approximately 1,000 participants. These trials demonstrated promising safety and immunogenicity results and were published in international peer-reviewed scientific journals. Following these initial phases, the Phase III clinical trials for Covaxin began in mid-November, targeting the recruitment of 26,000 volunteers across multiple sites in India. This was the largest Phase III efficacy trial ever conducted for any vaccine in India, marking India’s first and only Phase III efficacy study for a COVID-19 vaccine. The trials ensure the vaccine’s safety and efficacy before approval and widespread distribution.
What regulatory approvals has Covaxin received?
Covaxin has received several significant regulatory approvals to ensure safety:
- India: The Drugs Controller General of India (DCGI) granted conditional market authorization for Covaxin COVID vaccine in January 2022. This approval followed the recommendation of the Subject Expert Committee (SEC) of the Central Drugs Standard Control Organization (CDSCO) to upgrade the vaccine’s status from restricted emergency use, which had been granted in January 2021. The CDSCO also approved its sale or distribution for restricted use in emergencies of public concern.
- World Health Organization (WHO): Covaxin received emergency use approval from the WHO in November 2021. However, in April 2022, the WHO suspended the vaccine’s supply through UN procurement agencies due to deficiencies in implementing good manufacturing practices (GMP). Bharat Biotech responded by suspending the production of Covaxin for export to address these concerns.
- United States: In December 2020, Bharat Biotech partnered with US-based biopharmaceutical company Ocugen to collaborate on the clinical development, registration, and commercialization of Covaxin for the US market. In February 2022, the US Food and Drug Administration (FDA) lifted a clinical hold on Covaxin, allowing Phase II/III clinical trials to proceed in support of a biologics licence application (BLA) submission. However, in March 2022, the FDA declined to issue an emergency use authorization (EUA) for Covaxin for paediatric patients aged 2 to 18 years. Ocugen holds commercialization rights for Covaxin in North America and expanded these rights to include Mexico in April 2022.
- Other Countries: Covaxin has received emergency use authorization for adults in Mexico and is approved for emergency use in 20 other countries. Covaxin is licensed for use in 23 countries worldwide, although its distribution is predominantly within India, where over 77 million doses have been administered as of June 2022.
Why did the WHO suspend Covaxin’s supply?
The World Health Organization (WHO) stopped Covaxin supply for UN programs. This was because Bharat Biotech’s main manufacturing facility in Hyderabad didn’t fully follow good manufacturing practices (GMP). This happened because the facility had to focus entirely on making Covaxin due to the COVID-19 emergency. During this time, certain equipment needed for strict quality control wasn’t available due to the pandemic. Bharat Biotech emphasised that Covaxin’s quality was never compromised.
Furthermore, WHO has also mentioned that this does not raise concern over the safety and efficacy of the Covaxin. It remains safe and effective.
How does Covaxin work?
Covaxin is an inactivated vaccine developed from the SARS-CoV-2 virus, meaning it uses a virus that has been killed and cannot cause COVID-19. When you receive the Covaxin shot, your immune system recognizes the inactivated virus and produces antibodies against it. These antibodies help your body fight off the virus if you are exposed to it in the future. The vaccine also includes substances called adjuvants, which enhance the immune response and help provide longer-lasting immunity. Covaxin is easy to store, as it only needs refrigeration between 2℃ to 8°C.
How effective is Covaxin?
Covaxin has been shown to be 77.8% effective against symptomatic COVID-19 according to the final analysis of its Phase III trials. A booster dose six months after the second dose resulted in over 75% of participants having detectable neutralising antibodies, with even higher antibody levels than after the initial two doses. The booster also showed strong responses against the Omicron and Delta variants. Side effects are generally mild, including pain at the injection site and flu-like symptoms. Covaxin has also demonstrated strong safety and efficacy in children compared to adults.
What are the side effects of Covaxin?
Covaxin may cause mild side effects such as pain, swelling, redness, or itching at the injection site, as well as body ache, weakness, stiffness, nausea, vomiting, fever, malaise, and headache. These effects are typically temporary and resolve on their own.
However, severe side effects or consequences of Covaxin are rare but can include allergic reactions such as anaphylaxis. It’s crucial to seek immediate medical attention if you experience symptoms like difficulty in breathing, swelling of the face or throat, rapid heartbeat, or severe dizziness after vaccination. Additionally, while extremely rare, there have been reports of blood clotting disorders associated with Covaxin.
How long does it take for a side effect to occur after vaccination?
After vaccination, most people experience a sore arm, with more widespread effects like fever and chills usually appearing within 8 to 12 hours. These side effects usually resolve within 48 hours. Since the vaccine cannot cause a COVID-19 infection, experiencing symptoms indicates a healthy immune response. While rare, allergic reactions can occur within the first 15 to 30 minutes after the jab. More common side effects include arm soreness, redness, and swelling at the injection site, with body-wide effects lasting 12 hours or more. Experts advise that these side effects generally cease within 24 to 48 hours after vaccination, although slight fatigue or arm soreness may persist. Comparing 48 hours of side effects to the risk of hospitalisation and death from COVID-19, experts emphasise the benefits of vaccination outweigh potential side effects.
What was the reaction of the medical community regarding Covaxin’s approval?
The emergency approval of Covaxin before completing Phase III trials faced criticism from the Indian scientific community. Despite nearly 14 million COVID-19 cases, approval came as cases were dropping. The CDSCO’s vague term “restricted use in an emergency situation” left many puzzled.
Groups like the All India People’s Science Network called the approval “hasty,” while the All India Drug Action Network demanded transparency. Concerns grew after a Phase III trial participant died, with allegations of improper screening at the trial site.
However, 45 doctors, including former AIIMS directors, defended Covaxin, calling it India’s “gift to humanity” and labelling the criticism as “irresponsible.”
What should you do in case you have concerns regarding vaccines?
If you have specific concerns about health conditions post-vaccination, it’s advisable to consult with a healthcare professional. They can provide personalised medical advice and conduct any necessary evaluations. They can also help differentiate between vaccine-related issues and other potential causes of health problems. We would like to make it clear, one should not trust random and unreliable social media posts for making their healthcare decisions.
Are the authorities watching for heart problems after COVID-19 vaccines?
Yes, the CDC and other notable organisations have been actively monitoring COVID-19 vaccine-induced myocarditis. They provide transparent, evidence-based information on vaccine safety and participate in the WHO-led Vaccine Safety Net project. The CDC has launched investigations into myocarditis and pericarditis cases. Especially following mRNA vaccinations (Covaxin is an inactivated virus vaccine), with active surveillance in adolescents and young adults.
How does the CDC fight COVID-19 vaccine misinformation?
The CDC actively combats COVID-19 misinformation. To address false information on social media, the CDC uses a multimodal approach. It provides credible, evidence-based information on vaccine safety and adverse effects through its website and collaborates with health organizations. The CDC also uses social media to communicate with the public and dispel myths about COVID-19 vaccines.
We would like to conclude with, “The benefits of COVID vaccines far outweigh the associated side effects.“
We have debunked several claims regarding the COVID vaccines. Was COVID handling by the Government of India a huge scam? Has Japan’s government banned the COVID-19 vaccine? Has the German government admitted there was no Pandemic? Has Japan declared an emergency over the ‘explosion of mRNA cancers’? Are Covishield-vaccinated Indians susceptible to developing TTS?
(This fact check article was originally published by The Healthy Indian Project, THIP and has been republished by Lighthouse Journalism as a part of Shakti Collective )